Lilly’s COVID treatment sparks little demand
For a highly touted drug meant to keep throngs of people out of hospitals during a pandemic, Eli Lilly and Co.’s wonder treatment bamlanivimab sure has been slow to catch on.

For a highly touted drug meant to keep throngs of people out of hospitals during a pandemic, Eli Lilly and Co.’s wonder treatment bamlanivimab sure has been slow to catch on.
INCog BioPharma Services has purchased 16 acres of undeveloped land in Fishers for its planned new biopharmaceutical manufacturing facility. The $60 million project has grown in size.
The Sinopharm vaccine, like the AstraZeneca one, could be easier for countries around the world to handle since they can be stored at normal fridge temperatures.
The candidate made by Novavax Inc. is the fifth to reach final-stage testing in the U.S. Some 30,000 volunteers are needed to prove if the vaccine–a different kind than its Pfizer and Moderna competitors–really works and is safe.
Under the nearly $2 billion deal announced Wednesday, the companies will deliver at least 70 million additional doses by June 30, with the remaining 30 million to be delivered no later than July 31.
The lawsuit, filed Tuesday by the Justice Department, says the nation’s largest retailer did not properly screen prescriptions at its 5,000 pharmacies. The agency is seeking civil penalties that could total billions of dollars.
Red tape, staff shortages, testing delays and strong skepticism are keeping many patients and doctors from the drugs made by Eli Lilly and Co. and Regeneron Pharmaceuticals.
The Food and Drug Administration was evaluating a shot developed by Moderna Inc. and the National Institutes of Health and was expected to give it the green light soon, clearing the way for its use to begin as early as Monday.
Once FDA’s emergency use authorization is granted, Moderna will begin shipping millions of doses, earmarked for health workers and nursing home residents, to boost the largest vaccination effort in U.S. history.
The Food and Drug Administration said Wednesday that pharmacists can draw additional doses from vials of the Pfizer coronavirus vaccine, potentially expanding the country’s supply by millions of doses.
The vaccine appears poised for regulatory clearance after a detailed data review by Food and Drug Administration scientists confirmed the two-shot regimen was “highly effective” in a clinical trial and carried no serious safety concerns.

Lilly stock climbed Tuesday after the drugmaker laid out a better-than-expected revenue forecast and plans to buy a young company developing a potential Parkinson’s disease treatment.

Eli Lilly and Co. on Tuesday announced that it is buying New York-based Prevail Therapeutics Inc., an emerging player in the sizzling-hot area of gene therapies, which targets Parkinson’s disease and other brain-related maladies.
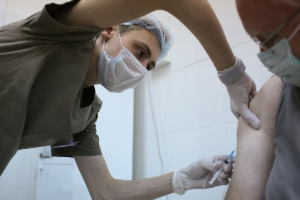
Federal officials hope to have given both of the required vaccine doses to 100 million people by the end of March. It could take two to three more months to immunize enough people to prompt herd immunity.

The state of Indiana is set to receive more than 55,000 doses of the COVID-19 vaccine for health care workers next week, with the initial doses going to five pilot hospitals. By the end of next week, additional doses are expected at a total of 50 hospitals throughout the state.

The U.S. gave the final go-ahead Friday to the nation’s first COVID-19 vaccine, marking what could be the beginning of the end of an outbreak that has killed nearly 300,000 Americans.
Doctors are reporting that a two-drug treatment involving a medication from Indianapolis-based Eli Lilly and Co. is especially helpful for COVID-19 patients who need extra oxygen.
Thursday’s meeting of the Food and Drug Administration’s vaccine advisory panel is likely the last step before a U.S. decision to begin shipping millions of doses of the shot, which has shown strong protection against the coronavirus.
British regulators warned Wednesday that people who have a history of serious allergic reactions shouldn’t receive the new Pfizer-BioNTech vaccine as they investigate two adverse reactions that occurred on the first day of the country’s mass vaccination program.
Trump administration officials denied that there would be availability issues in the second quarter, citing other vaccines in the pipeline, but others said problems are possible.