Indianapolis biotech trying to fight cancer navigates bumpy road
Point Biopharma is moving aggressively to get its lead drug, a radioactive isotope called lutetium 177, through late-stage trials for prostate cancer.
Point Biopharma is moving aggressively to get its lead drug, a radioactive isotope called lutetium 177, through late-stage trials for prostate cancer.
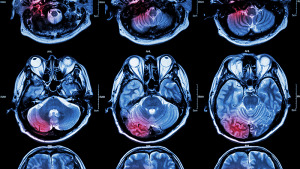
Three Indiana institutions are teaming up to try to develop a treatment for glioblastoma, a lethal cancer that begins with the brain or spinal cord, and is difficult to treat, often requiring a combination of surgery, chemotherapy and radiation.
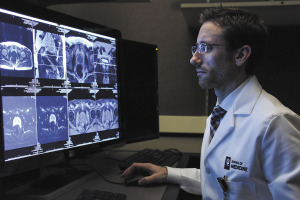
The imaging agent is the six-year-old company’s first commercial launch, following millions of dollars’ worth of research and clinical tests. It was approved by the U.S. Food and Drug Administration in December.
The medical school said the commitment will help launch research efforts to develop better therapies for triple negative breast cancer, an aggressive form of breast cancer that is often not responsive to hormone therapies and is resistant to chemotherapy.
Brooke Beier, senior vice president of commercialization at the Purdue Research Foundation, said FDA approval of the therapy is one of the most meaningful approvals ever for a Purdue-related innovation.
It’s a big moment for On Target Laboratories, an 11-year-old biotech based in West Lafayette. The FDA approval marks the company’s first novel compound to get across the finish line. The drug is marketed under the brand name Cytalux.

Indianapolis’ newest publicly company, Point Biopharma Inc., is the latest player in a field expected to see explosive growth as doctors and researchers look for new ways to shrink tumors.
Few such drugs are approved now, but the approach is predicted to become a new way to treat patients with other hard-to-reach or inoperable cancers.

Dee Alderman’s doctors told her in November, as cases heated up, to stay home completely; her husband and son decontaminate every time they come in the house.
Merus N.V. specializes is so-called CD3 engaging T-cell therapies, a growing area of cancer research, based on immunotherapy, or using the immune system’s T cells to find and shrink tumors

Just 12 years after opening to great fanfare, the future of the $150 million center, a partnership between the Indiana University School of Medicine and Indiana University Health, is full of questions.
The grant will help fund an ongoing study to evaluate long-term health outcomes for cancer patients who receive life-saving chemotherapy treatments that often have difficult side effects.

Hardesty, 51, said operations at his culinary business, Studio C, will be scaled back while he undergoes treatment over the next several months.
Estimates by the National Cancer Institute show there will be 10,000 more breast and colorectal cancer deaths over the next decade than would have been expected without the coronavirus. The director said that estimate was perhaps too conservative.
Development Corp., is helping raise money for a women-focused cancer research initiative. The campaign, which will run through June, is in its second year.

Indiana University Health’s new Schwarz Cancer Center is the latest addition to a crowded landscape of cancer centers and hospital oncology programs popping up around central Indiana.
Researchers on Wednesday reported the largest-ever one-year decline in the U.S. cancer death rate, a drop they credited to advances in lung-tumor treatments.
The drug, called pegilodecakin, had been seen as a promising treatment for one of the deadliest types of cancer, and was the lead product in Lilly’s $1.6 billion acquisition of Armo BioSciences last year.
The treatment costs $375,000 or $475,000, depending on whether it is used for advanced lymphoma or pediatric leukemia. Hospital stays can add hundreds of thousands of dollars to the cost of care.
The cancer center, opened in 2008, is now one of just 51 “comprehensive cancer centers” in the nation and the only one in Indiana.